11 Sep Optimizing All-Natural Nursery Feeds with ActiSaf® HR+ and NucleoSaf® 600
Dr. Joe Loughmiller – Phileo by Lesaffre, North American Senior Swine Technical Services Manager.

Why use Live Yeast Probiotics and Yeast Extracts in Nursery Feed?
Current consumer preference trends and increasing government regulation continue to drive interest in reduced antibiotic use and increased use of antibiotic alternatives, such as live yeast probiotics and yeast extracts like ActiSaf® HR+ and NucleoSaf® 600. ActiSaf® HR+ is a protected, proprietary strain of a saccharomyces cerevisiae live yeast probiotic for use in swine feeding programs. Usage is increasing as an effective, all-natural gut health and growth performance solution alternatives to feeding antibiotics and/or rendered blood products. ActiSaf® HR+ confers benefit to pigs through binding pathogenic bacteria, increasing cooperative activity of gut microbiota, reduced intercellular permeability, and immune stimulation (Posadas et al., 2017).
Using NucleoSaf® 600 free nucleic acids to support gut health during the weaning transition
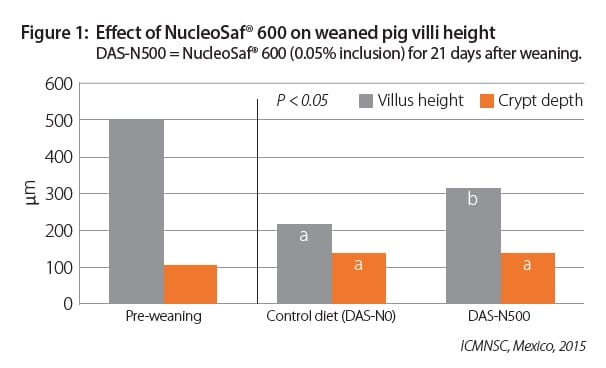
Nucleotides are required to optimize gut health and development, including immune function, microbiota development, enzyme expression and villi height (See Figure 1). These functions are critical to the proper growth and development of fast-growing pigs.
During stressful periods like weaning, feed intake drops, and nucleic acid supply decreases at a critical time of high growth potential and increased cellular activity. These feed intake changes
can increase the demand for free nucleotide supplementation from NucleoSaf® 600 to optimize performance and health. The fee nucleotides from NucleoSaf® 600 mean that the pig, during the weaning transition stress, can quickly and easily convert the nucleotide to nucleosides for maximum absorption and utility (See Figure 2). Conversely, other nucleotide rich ingredients and competitive products require several digestive steps from nucleotide-rich proteins in feed to free nucleosides before absorption (Hess and Greenberg, 2012).
Effects of ActiSaf® HR+ and NucleoSaf® 600 as part of an all-natural nursery feeding program
Trial 1: Pigs (n=700) were fed ABF nursery diets containing ActiSaf® HR+ and/or NucleoSaf® 600 and compared to pigs fed diets containing 0 or 4% spray-dried plasma during a 42-day nursery trial (PC=pos. control, NC=neg. control, AS=ActiSaf, NS=NucleoSaf, AS+NS=ActiSaf & NucleoSaf). There were 10 pigs/pen (avg. 13.9 lbs; ~20 d of age). Three phases were fed and performance, mortality and removals were measured.
Weekly average body weight differed between the PC and AS+NS treatments on d 14 and d 21, with pigs fed the AS+NS treatment being highest (P < 0.05; See Table 1). The heavier bodyweights were primarily due to higher feed intake. These results suggest that ActiSaf® HR+ and NucleoSaf® 600 can replace spray-dried plasma in weaned pig diets.
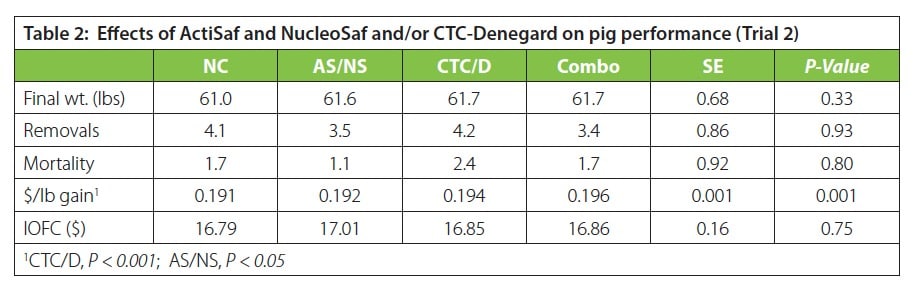
Trial 2: Pigs (n=4,320) were fed commercial nursery diets containing ActiSaf® HR+ and NucleoSaf® 600 with no medication or with CTC/Denegard medication for the first 21 days during a 42-day nursery trial (NC=neg. control, AS/NS=ActiSaf/NucleoSaf, CTC/D=CTC/Denegard; Combo=AS/NS & CTC/D). There were 27 pigs/pen (avg. 13.3 lbs; ~21 d of age). Three phases were fed and performance, mortality and removals were measured. Final body weight did not differ among treatments, but pigs fed the AS/NS treatment had similar final body weight as pigs fed CTC/D or Combo (P > 0.30; See Table 2). The heavier bodyweights were primarily due to numerically higher feed intake. Cost/lb gain was highest for pigs fed CTC/D or Combo, while pigs fed the AS/NS treatment were like pigs fed the NC treatment (P < 0.05). Of economical importance, pigs fed the AS/NS with or without medication numerically lower removals and mortality than either the CTC/D or NC treatments. These results suggest that pigs fed ActiSaf® HR+ and NucleoSaf® 600 have economically similar benefits as pigs fed a CTC/Denegard medicated feeding program.









Sorry, the comment form is closed at this time.